Oncology Clinical Development Expertise
Cancer Clinical Development is Complex, Our Experts Can Help.
Aspire Laboratories has extensive experience supporting >560 oncology projects and >70 oncology marketing applications in the United States, Europe, and Japan. We help oncology developers navigate the complexities of designing and executing their cancer trials in the most highly competitive and complicated market. Our extensive expertise includes traditional cancer treatments, devices/diagnostics as well as specialized therapies including vaccines, gene therapies, cell therapies, and immunotherapies .
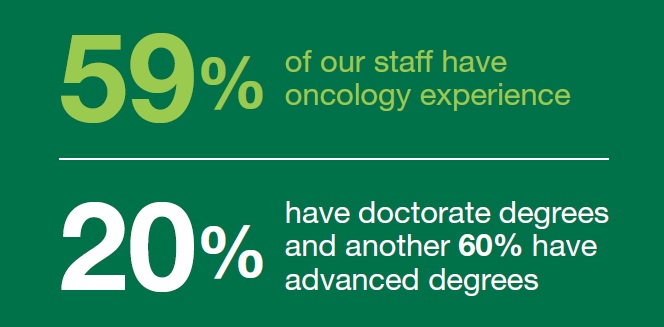
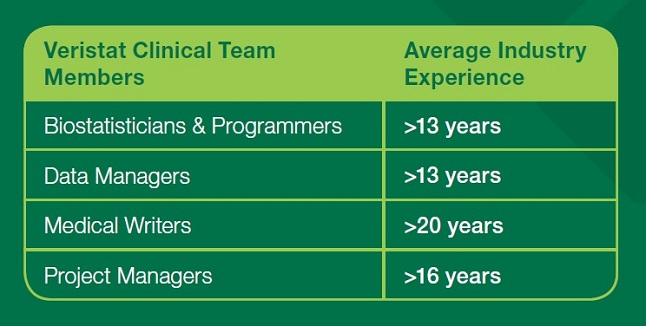
Oncology Experience
With cancer being the second* leading cause of death today, it’s no wonder that Aspire Laboratories clients are prioritizing their pipelines to develop life-extending cancer therapies. Aspire Laboratories teams work on a spectrum of amazing cancer treatments including drugs, vaccines, and diagnostics.
1. Cancer clinical development comprises >35% of the work we do
2. Cancer treatments account for 50% of the marketing applications that our teams have prepared,
3. In the past 5 years, 10 oncology products were approved for NDAs and jNDAs that we prepared
* Source: http://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm




